30+ fasenra eosinophil calculator
Learn About The Science Behind FASENRA. Ad Review Safety Efficacy Data From Clinical Studies For FASENRA.

Fasenra Benralizumab Mechanism Of Action For Hcps
Web FASENRA is indicated as an add-on maintenance treatment of patients 12 years and older with severe eosinophilic asthma.

. View Clinical Study Information. FASENRA is not indicated for treatment of other. WBC is the white blood count.
Web 30 mg subcutaneously once every 4 weeks for the first 3 doses then once every 8 weeks thereafter Comments. AbsoluteEosinophilCount WBC Eosinophils 100. WBC is the white blood.
Web Fasenra is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. Web Eosinophils percent Result Absolute Eosinophil Count Decimal Precision Notes This calculator is used for peripheral blood eosinophil count only. Web FASENRA is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people 12 years and older whose asthma is not.
Access Physician Information To Learn More About Prescribing FASENRA For Your Patients. Learn About The Science Behind FASENRA. Web Blood eosinophil unit conversion calculator Calculator Convert to obtain absolute eosinophil count in cellsμL.
The mechanism of action of FASENRA in asthma is not fully understood. Member has an absolute blood eosinophil count 150 cellsmcL within the past 3 months. -This drug should be injected into the upper arm.
Web FASENRA is a biologic designed to target and remove eosinophils a key cause of severe asthma. View Clinical Study Information. Learn More About FASENRAs Safety Efficacy Data.
Web FASENRA PEN 30 mgmL Solution for Injection FASENRA 30 mgmL Solution for Injection 2. Access Physician Information To Learn More About Prescribing FASENRA For Your Patients. QUALITATIVE AND QUANTITATIVE COMPOSITION Each FASENRA Pen.
The white blood cell WBC count and the eosinophil percentage can be used to estimate the total number of the acidophilic cells. Web This calculator is used for peripheral blood eosinophil count only. Learn More About FASENRAs Safety Efficacy Data.
Prescribed by or in consultation with a pulmonologist immunologist or. Web About Absolute Eosinophil Count. Ad Discover Over Two Years Of Safety Efficacy Info.
Reported eosinophil result Please confirm that your entry. Ad Discover Over Two Years Of Safety Efficacy Info. Ad Review Safety Efficacy Data From Clinical Studies For FASENRA.
Web The recommended dose of FASENRA is 30 mg administered once every 4 weeks for the first 3 doses and then once every 8 weeks thereafter by subcutaneous injection into the.

Results Clinical Review Report Benralizumab Fasenra Ncbi Bookshelf

G57001ku13i011 Gif

Benralizumab For Pdgfra Negative Hypereosinophilic Syndrome Nejm
Results Clinical Review Report Benralizumab Fasenra Ncbi Bookshelf

Fasenra Benralizumab Mechanism Of Action For Hcps

Ilgnlcdi55wlam

Benralizumab Efficacy By Atopy Status And Serum Immunoglobulin E For Patients With Severe Uncontrolled Asthma Sciencedirect

Baseline Blood Eosinophil Count As A Predictor Of Treatment Response To The Licensed Dose Of Mepolizumab In Severe Eosinophilic Asthma Sciencedirect

Fasenra Pen And Prefilled Syringe Dosing Fasenra Benralizumab For Hcps
Moa Blood Eosinophil Data Nucala Mepolizumab For Hes
Results Clinical Review Report Benralizumab Fasenra Ncbi Bookshelf
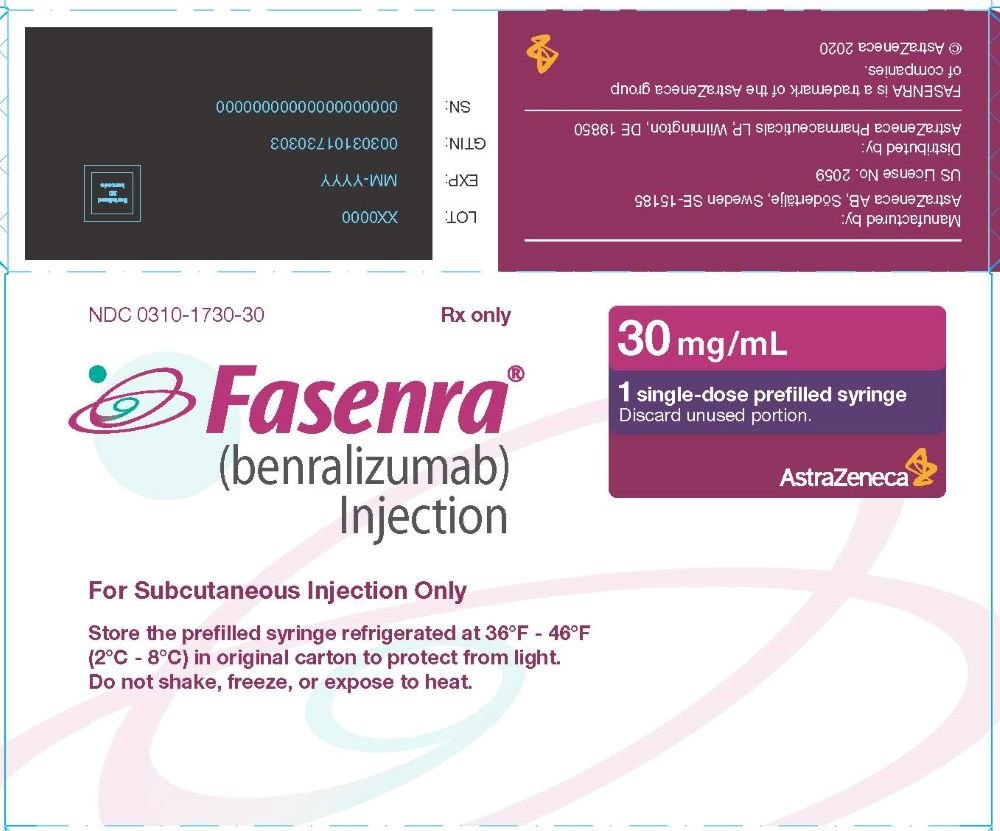
Fasenra Package Insert Prescribing Information Drugs Com

G57001ku07i005 Gif
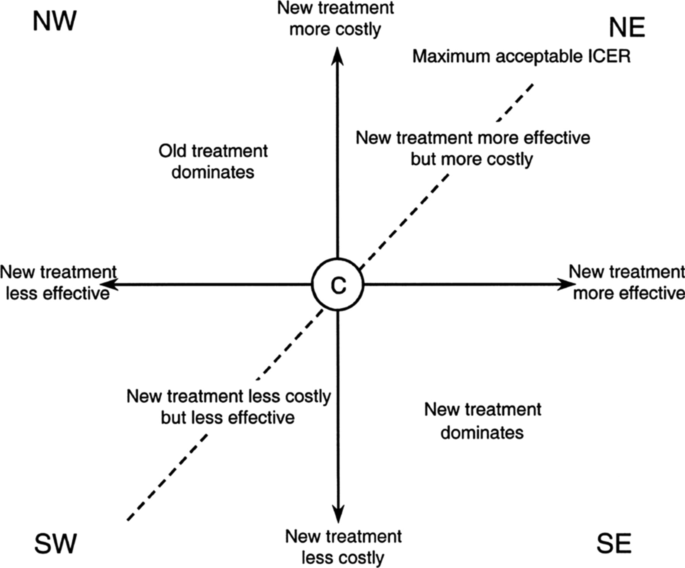
Real Life Cost Effectiveness Of Benralizumab In Patients With Severe Asthma Respiratory Research Full Text

Eosinophilic Asthma Treatment Information Fasenra Benralizumab For Hcps

How Do The Il 5 Inhibitors Cinqair Fasenra And Nucala Work Vasculitides A Vasculitis Blog
Fasenra Side Effects Dosage For Asthma And More